I. Introduction
Selling your own unique sauce can be a great way for you to grow your restaurant’s brand. However, the process can involve many steps, and can involve many government institutions. This primer can help you work through the various steps to ensure that you can get your sauce protected and out on the market.
II. Trademark Protection
The best way to protect your brand and new product is to obtain federal trademark protection from the United States Patent and Trademark Office (USPTO). By obtaining and maintaining a registered trademark(s), you can ensure that others cannot take advantage of the good will and brand loyalty you will work to build up in your brand.
You can obtain a registered trademark for several different aspects of your brand used with the product. You might trademark the name and/or logo you have chosen for your sauce, packaging, labels, or you may even decide to trademark an elaborate custom made bottle.
Once you have selected your branding and packaging, we will be able to help you apply for a federal trademark for the specific kind of goods you want to protect. For example, if you want to trademark the name of your sauce, we would trademark the name and its use for sauces. This would ensure not only that another merchant cannot use your brand to market a sauce, but that no one can use a confusingly similar brand/mark for similar products anywhere in the United States. The mark would offer you broad protection in the future, as you will be able to grow your brand nationally without worrying about similar marks conflicting with your own.
III. Registration with the U.S. Food and Drug Administration (FDA)
The FDA now requires registration of all facilities for manufacturing, processing, and packing food to register with the FDA. This registration requires renewal biannually, between October 1 and December 31 of even numbered years. Such registration includes an assurance that the FDA will be allowed to perform inspections of the facility.
The FDA registration can be completed online at https://www.access.fda.gov/. The form requires disclosure of the facility name, address, and telephone number, as well as contact information of the parent company, the owner or operator of the facility, and the contact information for a domestic agent if the company is international.
The FDA does have several exemptions to the registration requirement. For example, home kitchens are exempt, as are restaurants that do not package and sell goods to consumers. If you intend to bottle your sauce in your restaurant, you may qualify for an exemption as a restaurant or perhaps as a retail food establishment. A retail food establishment is defined as one which primarily functions to sell directly to consumers, so long as the annual amount of money made from sale to consumers exceeds the amount of annual money made from sales to others. Of course, this designation primarily includes grocery and convenience stores, so your restaurant’s ability to obtain an exemption may not be guaranteed.
It is advisable that you contact your local regional FDA office to be sure to fully comply with all of its requirements. The contact information for the Southwest regional branch can be found here: http://www.fda.gov/ICECI/Inspections/IOM/ucm124059.htm.
IV. Proper Labelling
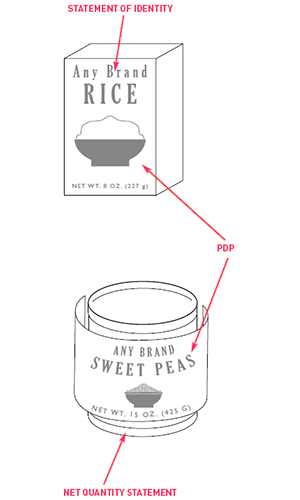
Figure 1
Food products sold within the United States are subject to specific labelling requirements by the Food and Drug Administration (FDA). You will need two labels, a front label and an information label, and each must contain several elements.
The front label, also known as the principal display panel (PDP), must display three elements, though these elements can be interrupted by pictures, logos, the name of your company, and the like. See Figure 1 for an illustration.
Firstly, you will want to place the fanciful name of your product (that is to say the brand name you are giving your sauce) prominently on the front label.
Secondly, you will also need to include the common name for sauce as well, which is best placed above or below the fanciful name. A common name is a name like barbecue sauce or hot sauce, rather the fanciful barbecue name.
Thirdly, you are required to provide a net quantity statement in the bottom 30% of the front label. This statement must indicate the amount of product contained within your container, which can be calculated by subtracting the weight of an empty bottle from the weight of a full bottle.
You will need to list the amount in both metric (milliliters) and U.S. measure (fluid ounces). For example, 16 fluid ounces would need to be indicated as “16 fluid oz. (473 ml).” Please keep in mind that the font you use for the label must meet the minimum size requirements based on PDP area (length x width of a square label or .40(height x circumference) for a cylindrical container). See Figure 2 for font requirements.
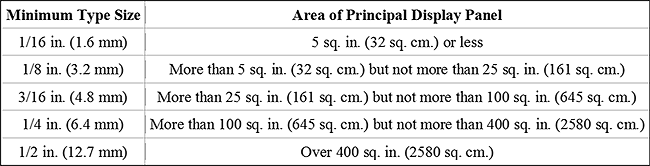
Figure 2
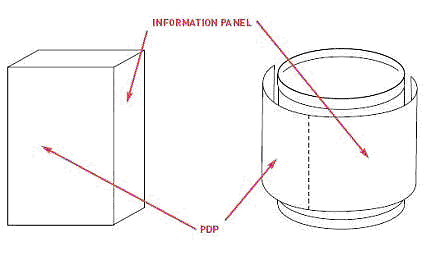
FIgure 3
You must also include an information panel on your container. This panel must be located to the right of the PDP (see Figure 3) and must also include at least three elements: the ingredients list, the contact information of your company, and, unless you are exempt, nutritional facts. Your font for this label should be clear and easy to read. The FDA recommends a type size at least 1/16″ inch in height (based on the lower case “o”), but font can be smaller for very small food packages. The information panel should be kept free of non-essential information, and no non-required information or artwork can divide the required information. The information on the information panel does not need to be perpendicular to the base or consistent in orientation, but you ought to strive to make the label as readable as possible.
The ingredients list must consist of all of the ingredients found in your product. These must be listed in decreasing order of prominence, from the most used ingredient (by weight) to the least. This includes water, if it is contained in your product. You must use the common name for the ingredient rather than a scientific or fanciful name. For example, use sugar instead of sucrose or Domino’s sugar. If chemical preservatives are used, you must give the common name of the preservative and its function, including a clause such as “to preserve flavour.” If you use artificial or natural flavors, you are allowed to merely described them and “Natural and Artificial Flavor.” If you use artificial colors, you will need to be aware if these colors are certified by the FDA, such as Red No. 40. You must list certified colors by name, but uncertified colors can be described merely as “artificial coloring.” If an ingredient is made of component parts, you must list the ingredient’s ingredients in parethesis.
If your ingredient list or any ingredients contain one or more of the major food allergens (milk, egg, fish, shellfish, tree nuts, wheat, peanut, or soybean) you are required to indicate such. You may either list these ingredients in parenthesis following the ingredient which contains the allergen (for example “whey (milk)”) or you may make a separate list below the ingredients list (Such as “Contains: milk, egg, and wheat”). Figure 4 illustrates these options best. If your product contains tree nuts, you must indicate which tree nut by using the tree nut’s common name (for example, cashew, chestnut, hickory nut, or pecans).

Figure 4
Secondly, the informational panel is also required to contain the contact information of your company. This means you will be required to list your company name, address (including street address, city, state, and zip code). You may also include telephone or fax numbers, and an e-mail address.
Thirdly, you may also need to include on the PDP or the informational panel a table of nutrition facts. If you employ less than 100 full time employees or sell less than 100,000 units in a 12 month period, you may be exempt from providing nutritional facts (See Figure 5 for other qualifications for exemptions).
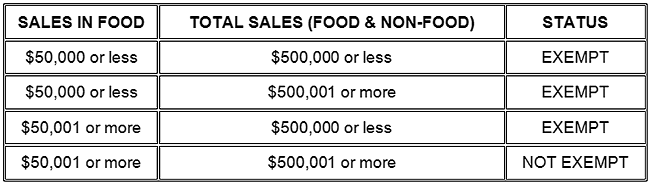
Figure 5
This exemption can be claimed by submitting, either electronically or by mail, the form locatable at the following link:
http://www.fda.gov/Food/GuidanceRegulation/GuidanceDocumentsRegulatoryInformation/LabelingNutrition/ucm053857.htm.
You do not need to claim your exemption with the FDA, but may choose to do so to get your exemption on record. Short of an exemption, you will need to have a laboratory analysis done on your product to determine the presence of the mandatory and voluntary nutrients, as required by the FDA. You will then need to list the required and voluntary nutrients found in Figure 6 in the proper format as described in Figure 7.
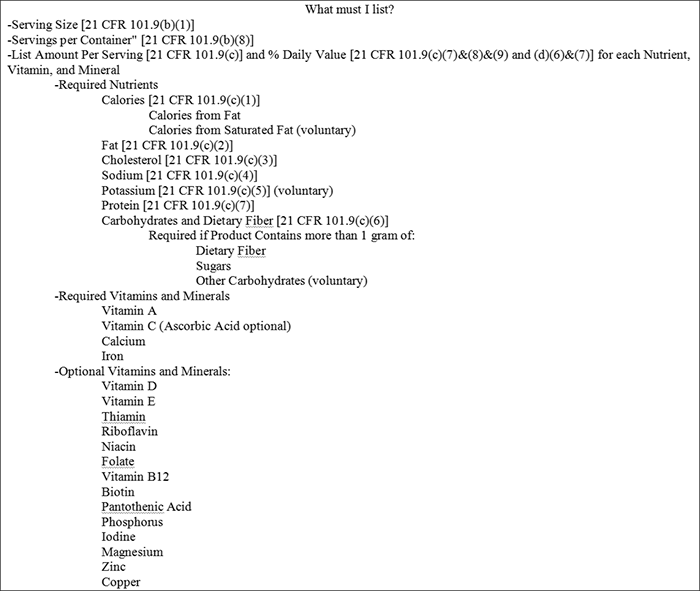
Figure 6

Figure 7
V. Local Permissions
Additionally, it is important to check with all state and county agencies to ensure compliance with local laws and requirements. If we can be of any assistance with helping you bring your new sauce or consumable to market, please contact Garcia-Zamor Intellectual Property Law, LLC







